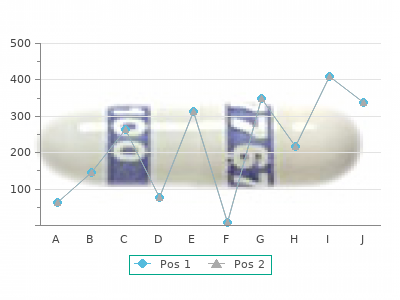
|
Download Adobe Reader
 Resize font: Resize font:
Kemadrin
By X. Yussuf. Pepperdine University. 2018. Other aspects of postnatal discount kemadrin 5mg overnight delivery, physical buy kemadrin 5 mg amex, functional and behavioural devel- opment were unaffected, except for a slight delay in vaginal opening by 1. The reproductive function of the F1 generation was normal, and the growth and development of the F2 generation were normal (Takahashi et al. Day-10 rat embryos [strain not specified] cultured for 24 h in vitro were exposed for the first 3 h to etoposide at concentrations of 1. A dose-related increase in the incdence of malformations was observed at doses of 2. The malformations observed consisted mainly of hypoplasia of the prosencephalon, microphthalmia and oedema of the rhombencephalon. Compa- rison of the concentrations necessary to produce 50% lethality and 50% malformations showed that amsacrine (see monograph, this volume) was 10 times and 20 times more potent, respectively, than etoposide. Etoposide has been reported to cause degeneration of rat spermatogonia and early spermatocytes, the appearance of large multinucleated spermatids and nuclear and cytoplasmic changes in Sertoli cells. Groups of three Sprague-Dawley rats, two to three months of age, were injected intraperitoneally with 5 or 10 mg/kg bw etoposide and killed 1, 3 or 18 days later. The effects of etoposide were most marked after one and three days, but some effects were still present 18 days after treatment (Hakovirta et al. In adult Sprague-Dawley rats injected intraperitoneally with a single dose of 5 or 10 mg/kg bw etoposide, it was a powerful inducer of micronuclei in early spermatids, whereas the major cytotoxic action is on the early spermatogonial stages. The highest dose caused marked depression of body-weight gain and food intake throughout gestation, and only two animals were alive at the end of the study. Deaths were observed at 3 mg/kg bw per day, and only 16/21 animals in this group were alive at termination. Doses up to and including 3 mg/kg bw had no adverse effect on embryo or fetal development, fetal weight, ossification or the incidence of malformations (Takahashi et al. The fetuses were removed for visceral and skeletal examination on day 28 of gestation. The body-weight gain of dams was depressed and liver damage was observed at the highest dose, but fetal survival and weight were unaffected and no gross malformations were observed. Histological examination of the fetal telencephalon showed no increase in cell death (Nagao et al. The second involves cytogenetic analyses of cells from patients with leukaemias associated with this treatment. An increased frequency of micronuclei was found in these samples compared with those obtained from untreated cancer patients or healthy, age-matched controls at a mean interval of 4. The regimens included up to six cycles of two daily oral doses of 50 mg/kg bw over 10–14 days separated by two weeks of rest. Taken together, these analyses suggest that etoposide is responsible for several non- random chromosomal translocations that are central to leukaemogenesis. It is also note- worthy that etoposide treatment of phytohaemagglutinin-stimulated human lymphocytes in culture is associated with an excess frequency of reciprocal translocations in addition to other abnormalities, which include dicentrics and, less often, unbalanced or complex rearrangements, deletions and inversions. Chromosomes 1, 11 and 17 are frequent targets of these abnormalities (Maraschin et al. Deletions or loss of chromosomes 5 and 7 were significantly asso- ciated with exposure to alkylating agents (p = 0. These are also the common translocations in de-novo cases of leukaemia; however, while translocations of chromosome band 11q23 are present in most cases of acute lymphoblastic leukaemia in infants and cases of monoblastic leukaemia in infants and young children, they are found in only about 5% of acute leukaemias in adults. More recently, chromosome band 11p15 was recognized as a site of recurrent translocations in leukaemias that follow etoposide-containing therapy (Stark et al. A group of 119 patients with advanced non-small-cell lung cancer were treated with four different cisplatin- based regimens, one of which contained etoposide; four patients developed acute myeloid leukaemia 13, 19, 28 and 35 months after the start of treatment, respectively. Three of the leukaemias had monoblastic features; in one case there was a t(9;11)(p22;q23) trans- location; in another there was t(9;11;18)(p22;q23;q12). The fourth case had a complex karyotype, with -5 and -7 abnormalities typical of alkylating agent-induced cases. Several additional case reports and cohort studies have indicated that, while the 9p22 locus is commonly involved in translocations with band 11q23 (Pedersen- Bjergaard et al. Observations of cases of undifferentiated leukaemia and acute lymphoblastic leukaemia with t(4;11)(q21;q23) suggested additional heterogeneity in the partner chromosomes involved in translocations with band 11q23 (Hunger et al. The translocation at band 11q23 was usually the only abnor- mality, but in five cases additional structural or numerical changes were detected. Trans- location of chromosome band 11q23 occurred in eight of the 10 cases, with fusions to band 9p21 or 9p22 in three cases and fusions to band 17q25, 19p13. Heterogeneous translocations involving band 11q23 were also observed in cases of leukaemia after epipodophyllotoxin-containing treatment for paediatric solid tumours.
When butter or agus may be canned with added water cheap 5 mg kemadrin mastercard, margarine is added 5 mg kemadrin amex, safe and suitable asparagus juice, or a mixture of both. When butter or margarine is agus juice is the clear, unfermented added, no spice or flavoring simulating liquid expressed from the washed and the color or flavor imparted by butter heated sprouts or parts of sprouts of or margarine is used. Cosmetic Act, or if it is a food additive (iv) Disodium guanylate complying as so defined, is used in conformity with the provisions of §172. I (4–1–10 Edition) material the quantity of stannous chlo- (g) The name of the food shall include ride added may exceed 15 parts per mil- a declaration of any flavoring that lion but not 20 parts per million cal- characterizes the product as specified culated as tin (Sn). Each of the in- more than sufficient to permit effec- gredients used in the food shall be de- tive processing by heat without discol- clared on the label as required by the oration or other impairment of the ar- applicable sections of parts 101 and 130 ticle. The food is sealed in a fied in paragraph (c)(1) of this section container and, before or after sealing, is present, the label shall bear the is so processed by heat as to prevent statement "lll oil added" or "With spoilage. The optional styles of the in with the common or usual name of mushroom ingredient referred to in the oil. The style as comparison of the prepared product, provided for in paragraph (a)(2) of this with the blended color produced by section shall be included as part of the spinning a combination of the fol- name or in close proximity to the name lowing concentric Munsell color discs of the food. Each of the in- lent of such discs: gredients used in the food shall be de- Disc 1—Red (5R 2. I (4–1–10 Edition) Disc 3—Black (N1) (glossy finish) (c) Salt means sodium chloride, deter- Disc 4—Grey (N4) (mat finish) mined as chloride and calculated as Such comparison is to be made in full percent sodium chloride, by the meth- diffused daylight or under a diffused od prescribed in "Official Methods of light source of approximately 2691 lux Analysis of the Association of Official (250 footcandles) and having a spectral Analytical Chemists," 13th Ed. Elec- shall be deemed in compliance for the tronic color meters may be used as an following factors, to be determined by alternate means of determining the the sampling and acceptance procedure color of tomato juice. Such meters as provided in paragraph (e) of this sec- shall be calibrated to indicate that the tion, namely: color of the product is as red or more (1) Quality. The quality of a lot shall red than that produced by spinning the be considered acceptable when the Munsell color discs in the combination number of defectives does not exceed as set out above. A lot shall be od prescribed in "Official Methods of deemed to be in compliance for fill of Analysis of the Association of Official container when the number of Analytical Chemists," 13th Ed. A collection of crose Solutions at 20°," which is incor- primary containers or units of the porated by reference. The total number 202–741–6030, or go to: http:// of sample units drawn for examination www. If no salt has been tion of the contents of a container, or added, the sucrose value obtained from a composite mixture of product from the referenced tables shall be consid- small containers that is sufficient for ered the percent of tomato soluble sol- the examination or testing as a single ids. For fill of container, the sample tionally or through the application of unit shall be the entire contents of the the acidified break, determine the per- container. Any sample unit shall specified in paragraph (c) of this sec- be regarded as defective when the sam- tion. Subtract the percentage so found ple unit does not meet the criteria set from the percentage of tomato soluble forth in the standards. The max- fractive index tables) and multiply the imum number of defective sample units difference by 1. The resultant value permitted in the sample in order to is considered the percent of "tomato consider the lot as meeting the speci- soluble solids. Such will be accepted approximately 95 per- juice may be homogenized, may be sea- cent of the time. Tomato the area of either Disc 3 or Disc 4; or 91⁄2 percent of the area of Disc 3 and 91⁄2 juice is the food intended for direct consumption, obtained from the percent of the area of Disc 4, whichever unfermented liquid extracted from ma- most nearly matches the appearance of ture tomatoes of the red or reddish va- the tomato juice. In the extraction of such combination, in addition to three de- liquid, heat may be applied by any fects for seeds or pieces of seeds, de- method which does not add water fined as follows, per 500 milliliters (16. Such juice is strained free fluid ounces): from peel, seeds, and other coarse or (a) Pieces of peel 3. I (4–1–10 Edition) (b) Blemishes such as dark brown or of this chapter, in the manner and form black particles (specks) greater than therein prescribed. A collection of primary con- ment of substandard quality specified tainers or units of the same size, type in §130. The number of primary substandard quality when the quality containers or units (pounds when in of the tomato juice falls below the bulk) in the lot. The total number of label may bear the alternative state- sample units drawn for examination ment, "Below Standard in Quality from a lot. A container, a por- the words specified after the cor- tion of the contents of a container, or responding paragraph (s) under para- a composite mixture of product from graph (b)(1) of this section which such small containers that is sufficient for tomato juice fails to meet, as follows: the examination or testing as a single (i) "Poor color". The maximum of fill of container for tomato juice, as number of defective sample units per- mitted in the sample in order to con- determined by the general method for sider the lot as meeting the specified fill of container prescribed in §130. The following accept- of this chapter, is not less than 90 per- ance numbers shall apply: cent of the total capacity, except when the food is frozen. Size container Lot size (primary container) (2) Determine compliance as specified n1 c2 in §156. Where the peas Vegetables are of sweet green wrinkled varieties, the name may include the designation §158. Anticoagulants order 5mg kemadrin with visa, Antithrombotics kemadrin 5mg free shipping, and Antiplatelets 265 Dosing Children: Initial S. Repeat anti-Factor Xa level before next dose and then 4 hours after the dose is administered If the anti-Factor Xa level is greater than 2Units/mL, then hold the dose until the anti-Factor Xa level has come down to 0. Repeat anti-Factor Xa level before the next dose until the level has come down to 0. Enoxaparin does not cross the placental barrier and does not bind to most heparin-binding proteins. Enoxaparin is renally excreted, 40% as active and inactive fragments and 10% as unchanged drug. Contraindications Contraindications to enoxaparin use are hypersensitivity to enoxaparin, heparin, pork products, or benzyl alcohol; patients with active major bleeding; and patients with thrombocytopenia. Use enox- aparin with caution in patients with any increased risk of hemorrhage, uncon- trolled hypertension, or renal impairment. Adverse Effects Potential adverse effects of enoxaparin use are edema, diarrhea, nausea, hematoma, normocytic hypochromic anemia, confusion, pain, dyspnea, fever, 11. Anticoagulants, Antithrombotics, and Antiplatelets 267 local irritation, atrial fibrillation, heart failure, eczema, skin necrosis, hemorrhage, thrombocytopenia, increased liver function tests, anaphylactoid reaction, hematoma, pneumonia, and pulmonary edema. Poisoning Information Therapeutic reference ranges for anti-Factor Xa levels are between 0. Protamine may be used at dosage of 1 mg of protamine for each milligram of enoxaparin admin- istered. Heparin Indication Heparin is indicated for prophylaxis and treatment of thromboembolic disorders. Inter- estingly, infants also have a decreased production of thrombin and have similar levels when compared with heparinized adults. Loading dose can be modified based on preexisting condition Systemic heparin adjustment: General guidelines: target range and dose will need to be adjusted based on clinical conditions. It is eliminated renally in small amounts as unchanged drug, with a half-life of 90 minutes and a range of 1 to 2 hours. Factors that can prolong the half-life include obesity, renal dysfunction, hepatic dysfunction, malignancy, infection, and the presence of pulmonary embolism. Contraindications Contraindications for heparin use are hypersensitivity to heparin or any com- ponent; uncontrollable active bleeding (unless secondary to disseminated 11. Anticoagulants, Antithrombotics, and Antiplatelets 269 intravascular coagulation); severe thrombocytopenia; and suspected or confirmed intracranial hemorrhage; shock, severe hypotension. Precautions/Warnings Preservative-free heparin should be used in neonates to prevent neonatal gasping syndrome. Drug/Drug Interactions Thrombolytic agents (urokinase, streptokinase) and drugs that affect platelet function (e. Adverse Effects Potential adverse effects of heparin are hemorrhage, thrombocytopenia, increased liver aminotransferase level, anaphylaxis, erythema, pain at the injection site, chest pain, fever, headache, chills, cutaneous necrosis with S. Discontinue all heparin if evidence of progressive immune thrombocytopenia occurs. Compatible Diluents/Administration Because of pain, irritation, and hematoma, it is recommended that heparin not be administered I. Dosing is weight based, however, patients weighing more than 110 kg should not receive doses greater than the recommended dose for a patient weighing 110kg (44mg I. The elimination half-life is ~10 minutes and the termimal half-life in healthy volunteers is 1. Contraindications Contraindications are hypersensitivity to hirudin products or any components of the formulation, and active major bleeding. Drug/Drug Interactions Thrombolytic agents (urokinase, streptokinase) and drugs that affect platelet function (e. Poisoning Information There are no specific antidotes for direct thrombin inhibitors. Treatment of lepirudin overdose is symptomatic and supportive, with management directed toward the control of bleeding. Compatible Diluents/Administration Reconstitute a 50-mg vial of lepirudin with 1mL of sterile water for injection or 0. For bolus infusions, further dilute lepirudin to a final concentration of 5 mg/mL and administer I. Protamine is also used to neutralize heparin during surgery or dialysis procedures. Mechanism of Action Protamine is a weak anticoagulant that combines with strongly acidic heparin to form a stable salt complex that neutralizes the anticoagulant activity of both drugs. Based on these dosing guidelines, Table 11-2 shows the appropri- ate dose of protamine to be administered according to the time that has elapsed since heparin was administered. Anticoagulants, Antithrombotics, and Antiplatelets 273 Table 11-2 Time since last dose Dose of protamine <30 minutes 100% of above dosing recommendations 30–60 minutes Administer 50–75% of dose 60–120 minutes Administer 37.
Changes in elec- tric charge and phospholipids composition in human colorectal cancer cells kemadrin 5mg with visa. Structural features of helical antimicrobial peptides: their poten- tial to modulate activity on model membranes and biological cells cheap 5 mg kemadrin free shipping. Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. Dathe M, Schumann M, Wieprecht T, Winkler A, Beyermann M, Krause E, Matsuzaki K, Murase O, Bienert M. Peptide helicity and membrane surface charge modulate the balance of electrostatic and hydrophobic interactions with lipid bilayers and biological membranes. Prediction of antibac- terial activity from physicochemical properties of antimicrobial peptides. A synergism between tem- porins toward Gram-negative bacteria overcomes resistance imposed by the lipopolysac- charide protective layer. Maqueda M, Sanchez-Hidalgo M, Fernandez M, Montalban-Lopez M, Valdivia E, Martinez-Bueno M. Diversity of entero- coccal bacteriocins and their grouping in a new classifcation scheme. Molecular characterization of genes involved in the produc- tion of the bacteriocin leucocin A from Leuconostoc gelidum. Natural antimicrobial peptides from bacteria: characteristics and potential applications to fght against antibiotic resistance. Structural and functional diversity of microcins, gene-encoded antibacterial peptides from enterobacteria. Microcin E492, a channel-forming bacteriocin from Klebsiella pneumoniae, induces apoptosis in some human cell lines. Antibacterial and antitumori- genic properties of microcin E492, a pore-forming bacteriocin. Effcacy of microcin J25 in biomatrices and in a mouse model of Salmonella infection. The cyclic structure of microcin J25, a 21-residue peptide antibiotic from Escherichia coli. Microcin J25 has a threaded sidechain-to-backbone ring structure and not a head-to-tail cyclized backbone. Structure of ther- molysin cleaved microcin J25: extreme stability of a two-chain antimicrobial peptide devoid of covalent links. Microcin J25 triggers cytochrome c release through irreversible damage of mitochondrial proteins and lipids. Molecular mechanism of transcription inhibition by peptide antibiotic Microcin J25. Effects of the antibiotic peptide microcin J25 on liposomes: role of acyl chain length and negatively charged phospholipid. The antibacterial action of microcin J25: evidence for disruption of cytoplasmic membrane energization in Salmonella newport. The role of bacterial membrane proteins in the internalization of microcin MccJ25 and MccB17. Biosynthesis, immunity, regu- lation, mode of action and engineering of the model lantibiotic nisin. The nisin-controlled gene expression system: con- struction, application and improvements. Subtilosin A, a new antibiotic pep- tide produced by Bacillus subtilis 168: isolation, structural analysis, and biogenesis. Genes of the sbo-alb locus of Bacillus sub- tilis are required for production of the antilisterial bacteriocin subtilosin. Structure of subtilosin A, an antimicrobial peptide from Bacillus subtilis with unusual posttrans- lational modifcations linking cysteine sulfurs to alpha-carbons of phenylalanine and threonine. Subtilosin production by two Bacil- lus subtilis subspecies and variance of the sbo-alb cluster. Isolation of the Bacillus sub- tilis antimicrobial peptide subtilosin from the dairy product-derived Bacillus amyloliq- uefaciens. Structure of subtilosin A, a cyclic antimicrobial peptide from Bacillus subtilis with unusual sulfur to alpha-carbon cross-links: formation and reduction of alpha-thio-alpha-amino acid derivatives. Membrane permeabilization, orientation, and antimicrobial mechanism of subtilosin A. Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Improvement of solubility and stability of the antimicrobial peptide nisin by protein engineering. Enhancement of the chemical and antimicrobial properties of subtilin by site-directed mutagenesis. Site-directed mutagenesis of the hinge region of nisinZ and properties of nisinZ mutants. CyBase: a database of cyclic protein sequences and structures, with applications in protein discovery and engineering.
Tis process may involve Harvesting of the leaves of the Aloe vera pasteurization cheap kemadrin 5 mg amex, ultraviolet stabilization purchase 5mg kemadrin, chemical plant is generally performed by hand, with the oxidation with hydrogen peroxide, addition of leaves cut from the base of the plant (Grindlay & chemical preservatives and additives, or concen- Reynolds, 1986). At the processing list showing the relative frequency of use of plant step, the leaves may be cleaned with water and ingredients within formulations fled with the a mild chlorine solution (Grindlay & Reynolds, United States Food and Drug Administration 1986). Consumers of products specifed in Section In 2006, the industry size for Aloe species 1. Most products were reported as conducted by the National Health and Nutrition derived from Aloe vera (90%). Working Group from publicly available data; due to the small use, the coefcient of variation is > 30%, so that the data for Aloe vera are less reliable than for other herbs]. It can be assumed that workers ingredient containing anthraquinones at no in the production of Aloe vera may be exposed, more than 50 ppm (Committee of Experts on as well as workers in pharmaceutical, cosmetic, Cosmetic Products, 2008). Tis maximum level and food industries that use Aloe vera as an was also demanded in a safety assessment of the ingredient. Te doses topical applications of control cream or creams of whole leaf extract were equivalent to average containing: 3% or 6% (w/w) gel; 3% or 6% (w/w) daily doses of approximately 0, 2. Te minimal erythema dose is defned as the minimal amount of radiation that causes 3. Te mice were killed afer a recovery/ of 48 male and 48 female F344/N rats were observation period of 12 weeks. Te doses of whole experiment, 1 year, was too short to consider this leaf extract were equivalent to average daily arm of the experiment as a full carcinogenicity doses of approximately 0, 0. Survival of exposed the incidence of skin neoplasms in any groups groups was similar to that of controls. Te free aglycone is then absorbed, the absorption, distribution, metabolism, or undergoes oxidation, and is excreted in the excretion of topically applied Aloe vera gel, whole urine as rhein, as was shown in three volunteers leaf extract or decolorized whole leaf extract in receiving Aloe vera or barbaloin (Vyth & Kamp, experimental animals or humans. Aloe vera latex contains radiolabelled acemannan at a dose of 20 mg/kg the anthrone C-glycosides aloin A (barbaloin) body weight (bw) per day for 3 months resulted and aloin B (isobarbaloin) that are linked by in peak blood concentrations at 4–6 hours and β-glycosyl bonds to D-glucopyranose. Of the administered dose, 73% Intracaecal administration of [14C]rhein to was excreted in the urine, with > 60% occurring male Wistar rats resulted in 37% of the radioac- within 24 hours; 13% of the material was found tivity being excreted in the urine and 53% in the in the faeces. Te highest tissue concentrations were labelled acemannan was converted to substances found in the kidney (De Witte & Lemli, 1988). Tese were char- acterized as aloe-emodin, rhein, an unidentifed aglycone, and conjugates of these aglycones. At early time-points (< 48 hours), a great majority of the radiolabel was associated with the gastrointestinal tract. Short-term studies were conducted in which At necropsy, no signifcant diferences were technical-grade acemannan (acemannan, 78%) observed between mice fed Aloe juice and the was fed to male and female Sprague-Dawley control mice. Likewise, histopathological exami- rats at doses up to 2000 mg/kg bw per day for nation of the livers revealed no treatment-related 6 months, and to male and female beagle dogs at lesions (Sehgal et al. Male and female Wistar Hannover rats fed Tere were no signifcant gross or microscopic whole leaf powder from Aloe arborescens Miller lesions in either species that could be associated var. Rats from the 2-year study also showed 7 months [corresponding to doses of Aloe vera pigmentation, epithelial thickening, and atypical gel of ~0. None of the treatments caused any an Aloe vera decolorized whole leaf extract to obvious histopathological changes (Ikeno et al. Te same Aloe preparation was also Aloe vera decolorized whole leaf extract (aloin mixed in the diet at a concentration of 100 g/kg A, 0. Aloe vera whole examination of the caecum, colon, and rectum leaf extract decreased transit times in the rats, but indicated no signifcant alterations (Sehgal et al. Aloe vera Liliaceae was extracted with 95% ethanol, the solvent was evaporated, and the 4. Mice treated with the Aloe vera Upon oral ingestion, Aloe vera components preparation had an increased incidence (P < 0. Likewise, intestinal microfora appears to be dependent upon the presence of metabolize acemannan to smaller compounds by the anthraquinone fraction, in particular aloe- cleavage of the β-1→4 linkages. Aloe vera did not display any treatment-related patholog- gel, decolorized gel, or decolorized whole leaf). Aloe-emodin also contains a benzylic bacterial assays for mutagenesis and/or other hydroxy moiety that has the potential to undergo assays for genotoxicity. Tese data suggest that the Te impact of this increased production is pres- neoplastic response observed with Aloe vera is a ently not clear. Although the mechanism by consequence of the conversion of the anthrone which Aloe vera whole leaf preparations induce C-glycosides to aloe-emodin, which by itself or intestinal neoplasms in rats is not fully under- in combination with other Aloe vera components stood, it is clear that the molecular pathways is responsible for the development of adenomas observed in the intestinal neoplasms induced in and carcinomas in the large intestine. Summary of Data Reported mice did not develop adenoma or carcinoma of the large intestine, which may be due to the fact 5. Te leaves contain two types of liquids: a shorter gastrointestinal tracts and faster gastro- yellow bitter latex under the skin, and a viscous intestinal transit times than rats, which could gel in the inner section. Commercial products are contribute to the lack of a tumour response in made from processed leaves. Decolorization removes emodin cream on the photocarcinogenic activity pigments and anthraquinones from the whole of simulated sunlight in female mice based on leaf extract. Kemadrin
8 of 10 - Review by X. Yussuf Votes: 331 votes Total customer reviews: 331 |
|